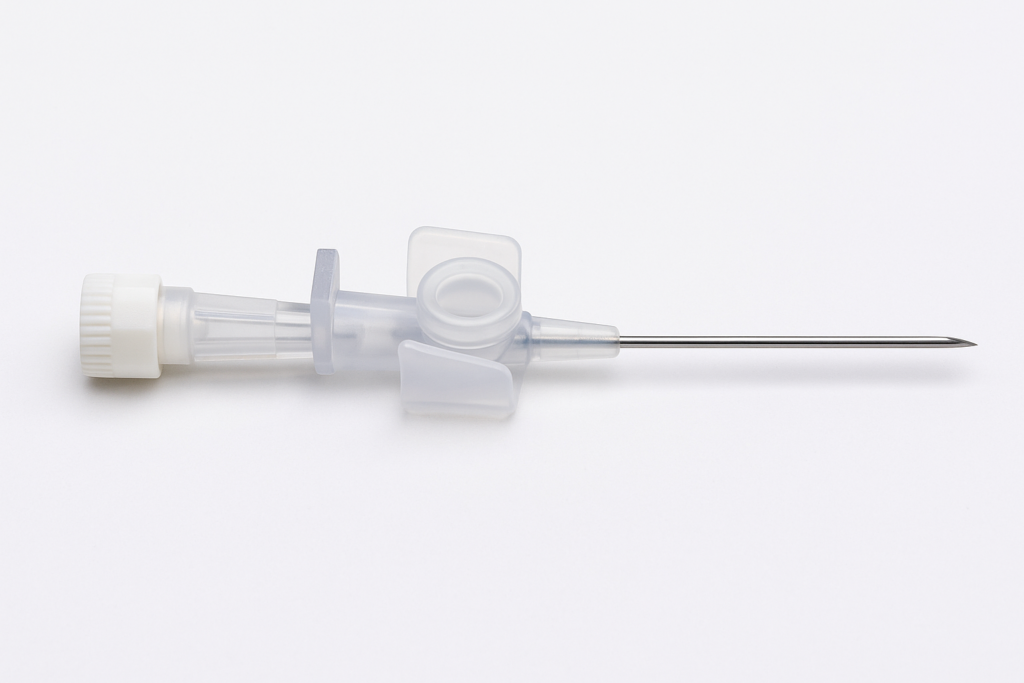
In the indwelling needle system, the check valve is a tiny yet critical component. Though simple in structure, it directly determines the stability of functions such as blood return blocking, infusion backflow prevention, and pipeline sealing. In recent years, a growing number of medical device manufacturers have begun adopting Liquid Silicone Rubber (LSR) for the production of check valves in indwelling needles, replacing traditional TPE or butyl rubber solutions.
Ⅰ、Practical Reasons for the Material Transformation of Check Valves in Indwelling Needles
In indwelling needle products, the check valve is required to achieve precise opening and closing under micro-pressure differences, while maintaining airtightness and elastic stability through multiple cycles. Although traditional TPE valve discs are easy to mold and low in cost, they have several long-standing issues that have plagued manufacturers:
- After multiple sterilizations, the rebound performance degrades, leading to unstable sealing;
- The surface is prone to adhesion and has a high friction coefficient, which affects the sensitivity of opening and closing;
- There are slight leaching or odor issues when in contact with medical fluids.
Liquid silicone rubber (LSR) offers distinct advantages in addressing these pain points: it features a stable molecular structure and high chemical inertness, enabling resistance to high-temperature steam sterilization, ethylene oxide (EO) sterilization, and gamma-ray irradiation; its high rebound rate (≥95%) ensures long-term sealing performance; it boasts high transparency and no odor, complying with ISO 10993 and USP Class VI biocompatibility standards.

Ⅱ.Performance Differences Between Liquid Silicone Rubber (LSR) and Traditional Materials
In the check valve of an indwelling needle, even minor differences in material performance often determine the safety level of the entire infusion system. Compared with traditional materials such as butyl rubber and TPE (thermoplastic elastomers), Liquid Silicone Rubber (LSR) offers distinct advantages in key indicators including high-temperature sterilization resistance, sealing and rebound performance, cleanliness, and dimensional stability.
Performance Dimensions | Butyl Rubber | TPE (Thermoplastic Elastomer) | Liquid Sillicone Rubber(LSR) |
High-Temperature Sterilization Resistance | Prone to aging and yellowing at high temperatures | Deformation after prolonged sterilization | Stable and non-discolorable, suitable for multiple sterilizations |
Rebound and Sealing Performance | Rapid rebound degradation | Unstable thrust | Durable elasticity and consistent sealing |
Biocompatibility | Leaching prevention required | Migration risk exists | Compliant with ISO10993 / USP Class VI |
Production Cleanliness | Vulcanization required, prone to residues | May contain additives | Solvent-free, one-piece molding |
Storage Stability | Prone to adhesion and aging | Prone to hardening | Long-term stability without deformation or adhesion |
Dimensional Accuracy | Large molding error | Average molding consistency | Injection molding accuracy of ±0.02mm |
Compatibility with Sterilization Methods | EO gas only | EO gas compatible, but not resistant to high temperatures | Compatible with multiple modes: steam, gamma-ray, and EO gas |

Ⅲ.Advantages of Liquid Silicone Rubber (LSR) Injection Molding in the Production of Check Valves
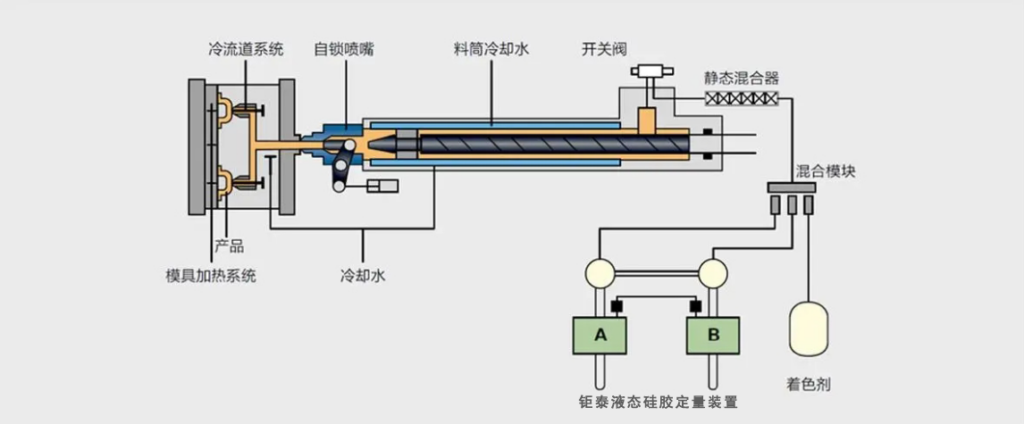
The liquid silicone rubber (LSR) check valve adopts the LSR injection molding process (Liquid Injection Molding, LIM), which is different from traditional compression molding or hot runner TPE injection molding. It achieves one-shot molding through two-component automatic mixing + vacuum injection + high-temperature curing, with the following distinct advantages:
1.High dimensional accuracy: The thickness of the valve disc can be controlled within ±0.02mm;
2.Excellent batch consistency: The automatic metering system ensures a constant mixing ratio;
3.No residual contamination: No release agents or vulcanizing agents are required;
4.High-yield production: Automatic demolding without trimming, compatible with automatic assembly lines.
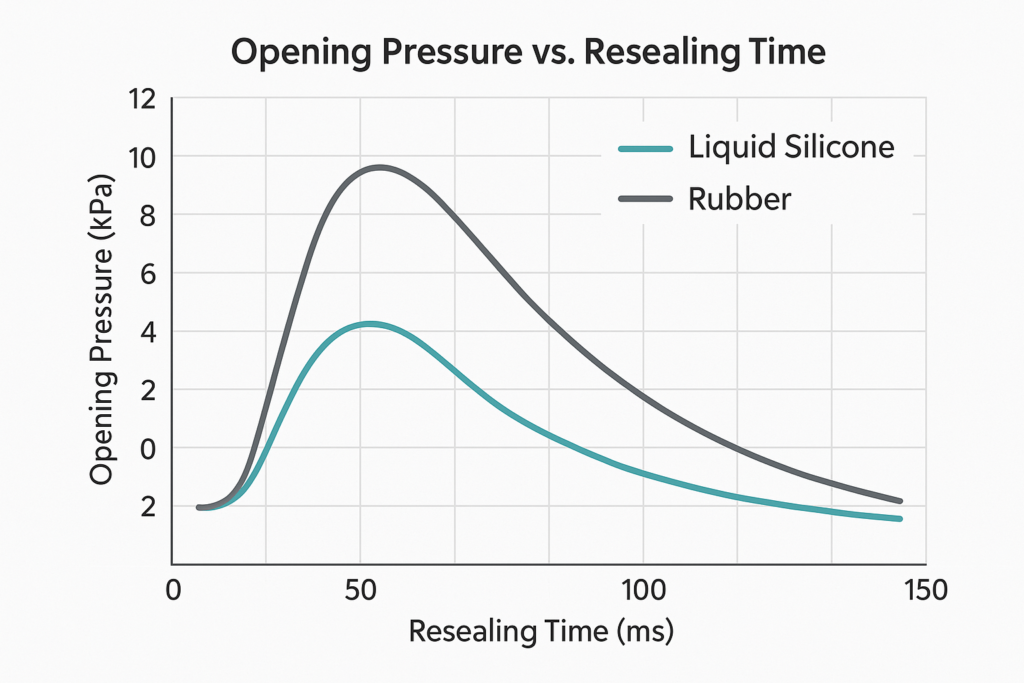
Ⅳ.Performance Verification and Application Performance
the perspective of structural design, the key to a check valve lies in the balance between the opening/closing force of the valve disc and its sealing rebound. Under the same conditions, the opening/closing pressure curve of the liquid silicone rubber (LSR) valve disc is smoother with a narrower controllable range:
- Opening pressure: 2~5 kPa (adjustable according to the formula);
- Rebound time: Less than 50 ms;
- Leakage rate: <0.001 mL/min;
- Performance retention rate after multiple sterilizations: ≥95%
V.Industry Trend: From Material Upgrade to System Optimization
The medical device industry is undergoing a transition from “functional usability” to “standardization.” Whether it is disposable indwelling needles, PIVC catheters, or automatic infusion systems, the requirements for valve components are evolving toward “traceability, sterilizability, and repeatable verification.”
The high purity and high stability of liquid silicone rubber (LSR) have enabled it to gradually replace traditional rubber materials, becoming the mainstream material for medical valves, seals, and fluid-stop components.
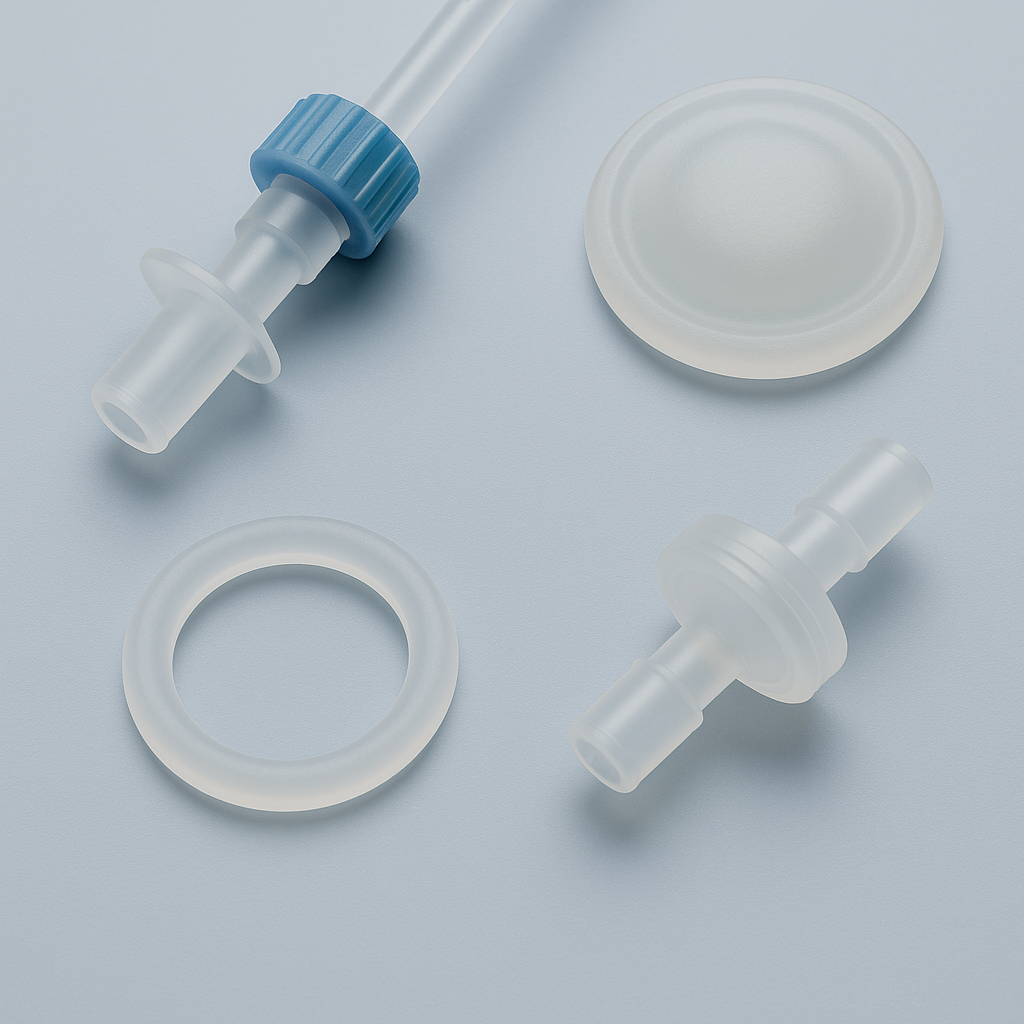
VI.Conclusion: From Material Upgrade to Capability Enhancement
The upgrading and replacement of indwelling needle check valves is not merely a change in materials, but more importantly, an evolution of production concepts and quality control systems. The introduction of liquid silicone rubber (LSR) has transformed valve component production from reliance on manual experience to data-driven and traceable manufacturing, which represents a technical efficiency improvement for the entire medical consumables industry.
Amid this trend, Jutai Silicone has continued to deepen its expertise in medical liquid silicone rubber (LSR) molding technology, microstructured mold design, and clean production systems. It has provided customized manufacturing solutions—including indwelling needle valves, infusion connectors, and catheter seals—for numerous medical device enterprises.
If you are planning to upgrade valve component precision, improve sterilization stability, or promote the mass production of liquid silicone rubber (LSR) molding, please feel free to contact Jutai Silicone. We will leverage professional solutions to help you achieve more efficient production and quality improvement.